What is lithium?
- Lithium’s chemical symbol is Li
- A soft silver-grey metal
- Third element in periodic table
- Member of the alkali group of metals
- Used in batteries, glass, ceramics, lubricants
- Lithium is a specialty industrial product bought and sold under contract, and the chemistry is specifically tailored to the customer’s needs
Why lithium-ion batteries?
- Highest output of energy / unit of weight
- Last longer because of the increased density of their cells
- Longer runtime on a single charge
- No memory of lazy battery effect
What are its uses & applications?
Application |
2014 Market Size |
Growth Rate |
Products |
|
| Traditional Use | Glass/Ceramics | 46KT | GDP | •Spodumene •Li2CO3 |
| Greases/Lubricants | 18KT | GDP | •LiOH | |
| Chemical Synthesis | 11KT | GDP | •Li Organometallics fed by Li Metal LiCl | |
| Energy | Portable Electronics & Other Handhelds | 48KT | 16% (Base Case) | •BG Li2CO3 •BG LiOH •BG Li Metal •BG Electrolyte Salts •BG LiCl •BG Alloys •BG Specialty Compounds |
| Hybrids | ||||
| Battery Electric Vehicle (BEV) | ||||
| Grid and Other Power Storage Applications |
Geographical Sources
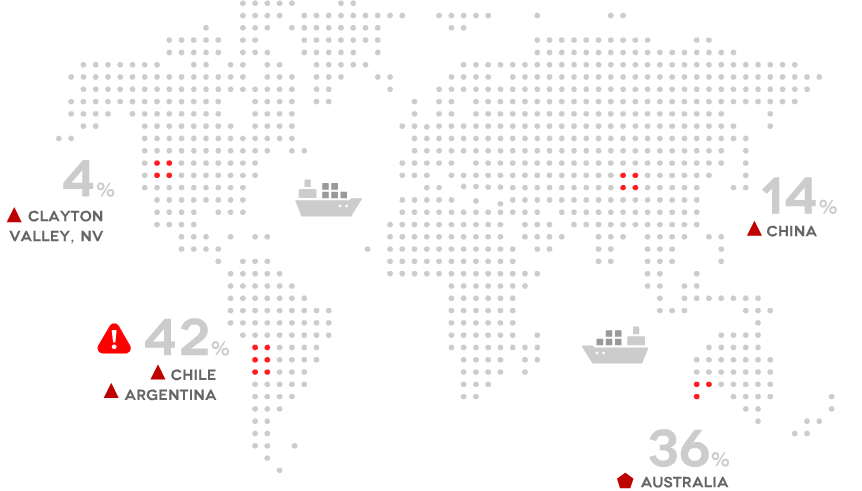
What are the different types?
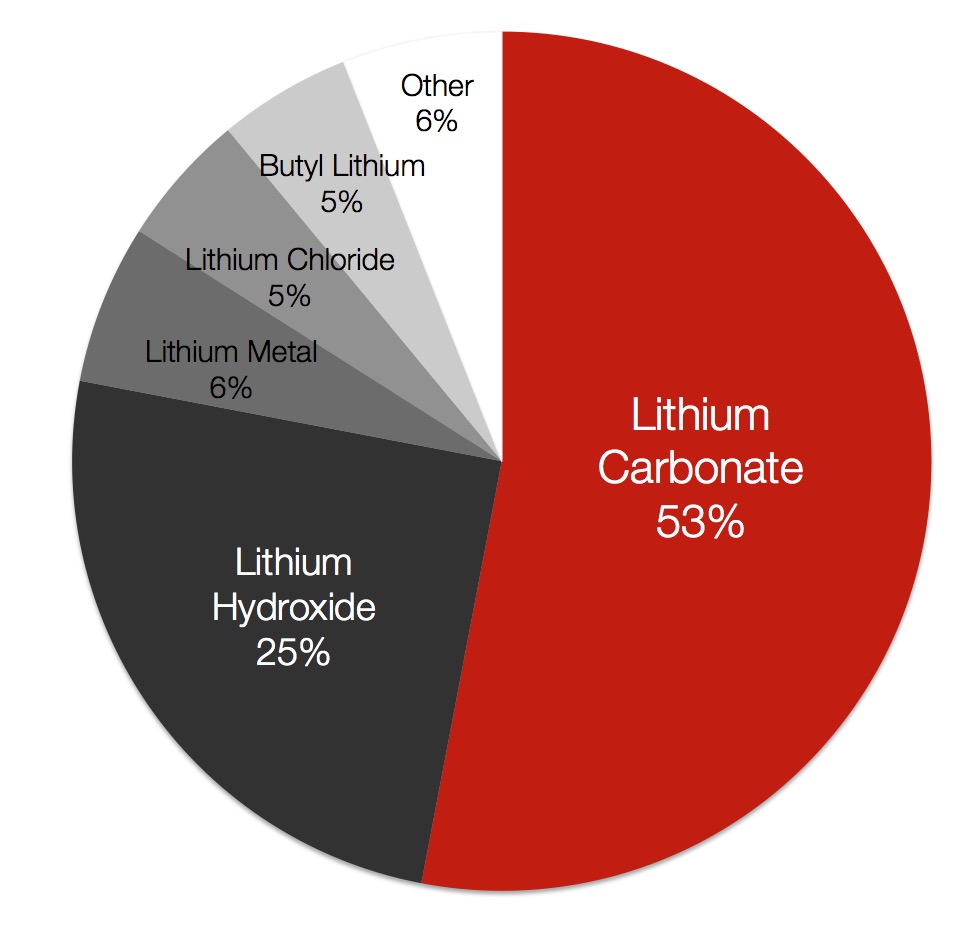
Lithium hydroxide and lithium carbonate are both consumed in battery cathodes. The high energy content and light molecular weight of lithium makes it an ideal energy source for transportation.
Geological Sources
Brine: 61%
- Lithium brine bodies in salt lakes, or salars, are formed in basins where water that has leached the lithium from the surrounding rock is trapped and concentrated by evaporation
- The process of extracting the lithium from brines involves pumping the brines into a series of evaporation ponds to crystallize other salts, leaving lithium-rich liquor
- Shallow wells
- This liquor is further processed to remove impurities before conversion to either lithium carbonate or lithium chloride for further upgrading to lithium hydroxide
Hard Rock: 39%
- There are three lithium minerals commercially mined today: spodumene, petalite and lepidolite
- Spodumene is the most important commercially mined lithium mineral given its higher inherent lithia content
- Both open-pit and underground mining methods are used to extract lithium minerals
- Once extracted, the lithium mineral ore is crushed and subjected to a number of separation processes to upgrade the lithium content by removing waste materials
Lithium Industry Fundamentals
Market Demand and Supply
- Acknowledged demand growth
- Batteries have powered gadgets for decades. They have now started to power cars, and pretty soon will be helping to keep the lights on, opening up a “massive” and “untapped” opportunity for investors, according to Goldman Sachs.
- Key Demand Drivers:
- Insufficient supply growth
- Current hard-rock and brine projects are capacity constrained.
- Furthermore, many brine sources are located in areas that are, geopolitically speaking, less than ideal.
- The U.S. has only one commercially producing operation and imports 80% of lithium it consumes, despite lithium being considered a strategic metal.
- Lithium accounts for roughly 2% of the cost of lithium-ion batteries
- Thus, the price of lithium can increase dramatically without becoming particularly noticeable to buyers of consumer electronics, electric vehicles or large storage batteries.
- Prices are projected to rise
- Prices have tripled since 2003
- FMC recently announced an “across-the-board 15% increase in price.”
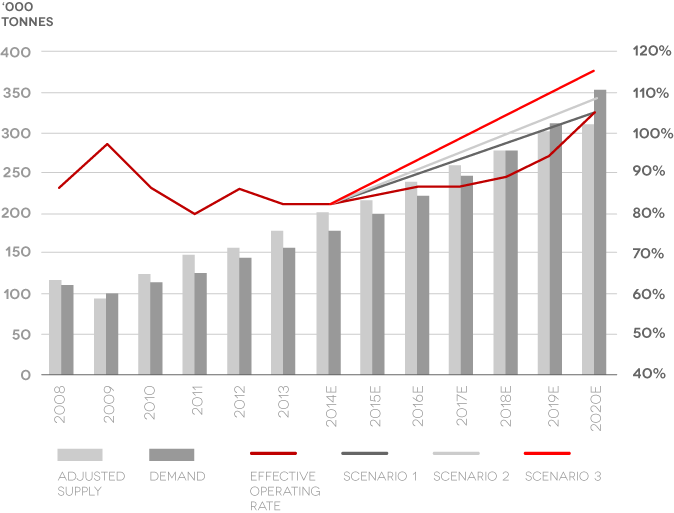
Source: Credit Suisse, 2104
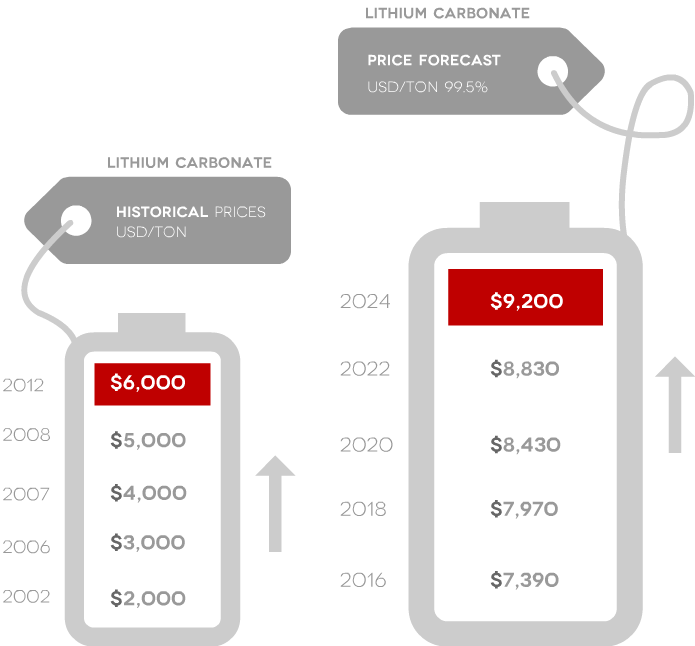
Source: Roskill and Signumbox and Stormcrow
New Solvent Extraction Process
Leveraging Modern Technology
- Faster (and not weather dependent)
- Higher purity
- Sustainable and more environmentally friendly
- Compact and Cheaper
Candidate Partners:
- Tenova Bateman
- POSCO
- OUTOTEC
- Stria
- Simbol
- Enirgi



